Nothing is safe: Intolerance of uncertainty is associated with compromised fear extinction learning
By Jayne Morriss, Anastasia Christakou, and Carien M van Reekum
Letter 8 Resource: Link to Article
Abstract
Extinction-resistant fear is considered to be a central feature of pathological anxiety. Here we sought to determine if individual differences in Intolerance of Uncertainty (IU), a potential risk factor for anxiety disorders, underlies compromised fear extinction. We tested this hypothesis by recording electrodermal activity in 38 healthy participants during fear acquisition and extinction. We assessed the temporality of fear extinction, by examining early and late extinction learning. During early extinction, low IU was associated with larger skin conductance responses to learned threat vs. safety cues, whereas high IU was associated with skin conductance responding to both threat and safety cues, but no cue discrimination. During late extinction, low IU showed no difference in skin conductance between learned threat and safety cues, whilst high IU predicted continued fear expression to learned threat, indexed by larger skin conductance to threat vs. safety cues. These findings suggest a critical role of uncertainty-based mechanisms in the maintenance of learned fear.
1. Introduction
The ability to discriminate between threat and safety is crucial for survival. Through fear conditioning, an organism can associate neutral cues (conditioned stimulus, e.g. a visual stimulus such as a shape) with aversive outcomes (unconditioned stimulus, e.g. shock, loud tone). Repeated presentations of a neutral cue with an aversive outcome can result in fearful responding to the neutral cue alone (conditioned response). This learned association can also be extinguished by repeatedly presenting the learned threat cue without the aversive outcome, a process known as fear extinction (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Milad & Quirk, 2002; Phelps, Delgado, Nearing, & LeDoux, 2004). During fear extinction, a reduction in reactivity to the learned threat cue over time is thought to reflect changes in harm expectancy and contingency beliefs (for a review see, (Hofmann, 2008)). Such fear extinction processes, however, are thought to be disrupted by cognitive biases – including attentional and expectancy biases – in individuals with anxiety and trauma disorders (Aue & Okon-Singer, 2015), who display delayed fear extinction or even extinction-resistant fear (Graham and Milad, 2011, Milad and Quirk, 2012, Mineka and Oehlberg, 2008). For example, compared to healthy controls, patients show elevated autonomic nervous system activity to both learned threat and safety cues at the start of extinction, and to learned threat cues across fear extinction learning (Blechert, Michael, Vriends, Margraf, & Wilhelm, 2007; Michael, Blechert, Vriends, Margraf, & Wilhelm, 2007; Milad et al., 2008, Milad et al., 2009).
In addition to examining fear extinction processes in clinical samples, it is important to test individual differences in non-clinical samples, to appropriately separate those processes that are risk factors for anxiety disorder development from those processes that are consequential to an anxiety disorder (Chambers, Power, & Durham, 2004). In two recent meta-analyses, however, only small differences in fear extinction behavior were found between anxious and non-anxious individuals (Duits et al., 2015, Lissek et al., 2005). Furthermore, findings have also been mixed from studies examining fear extinction behavior and trait anxiety, as measured with the Spielberger State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). For example, trait anxious individuals have been shown to display slower reductions in startle reactivity to both threat and safety cues during extinction (Gazendam, Kamphuis, & Kindt, 2013), but not in skin conductance (Haaker et al., 2015) or expectancy ratings (Barrett and Armony, 2009, Gazendam et al., 2013). These equivocal findings may stem from a lack of alignment between the STAI measure and the underlying cognitive mechanisms that disrupt fear extinction. For example, items in the STAI broadly address physical fear and anxiety symptoms or worrying, but items in the STAI do not capture any specific elicitors of fear and anxiety that may be related to fear extinction processes, such as harm expectancy or contingency beliefs.
Only very recently has research begun to assess the role of intolerance of uncertainty (IU) in fear extinction (Dunsmoor, Campese, Ceceli, LeDoux, & Phelps, In press; Morriss, Christakou, & van Reekum, 2015). IU is defined as a dispositional tendency that affects how uncertain situations are perceived and interpreted. Individuals with high IU scores tend to find uncertain situations inherently aversive and anxiety provoking. During experienced uncertainty, high IU individuals may be prone to distorted contingency beliefs, where the expectancy of threat may be disproportionate to the expectancy of safety. This may result in the generalization of potential threat to ambiguous, neutral, or even positive cues (Dugas, Buhr, & Ladouceur, 2004). Originally, IU was considered to be specifically related to Generalized Anxiety Disorder (Dugas et al., 2004). However, growing evidence suggests IU may be a transdiagnostic factor across many anxiety and mood disorders (Carleton, Fetzner, Hackl, & McEvoy, 2013; Gentes and Ruscio, 2011, McEvoy and Mahoney, 2012). Furthermore, the development of new disorder-specific IU scales (Thibodeau et al., 2015) highlights that IU may be applicable to specific phobia and Post-Traumatic Stress Disorder (PTSD), which are associated with compromised fear extinction learning.
In the context of fear extinction learning, uncertainty surrounding unannounced learned contingency changes (i.e. CS-US pairings) may initiate generalized expectancy of potential threat in high IU individuals, resulting in fearful responding to both learned threat and safety cues. In a recent neuroimaging study, during early fear extinction learning, we found high IU scores to be associated with equally high skin conductance to learned threat and safety cues, as well as greater activity within the right amygdala to learned safety vs. threat cues, suggesting threat generalization. Furthermore, in late extinction learning, high IU scores were associated with continued fear expression to learned threat vs. safety cues, indexed by larger skin conductance and right amygdala activity (Morriss et al., 2015). Given these recent findings outlined above, it seems pertinent to further examine whether IU proves to be a more sensitive predictor of compromised fear extinction, over general trait anxiety measures such as the STAI. Understanding associations between IU and fear extinction learning could help characterize specific IU-related cognitive biases that disrupt fear extinction processes, such as expectancy of potential threat that may impede the re-establishment of a previously paired CS+ as safe, with implications for targeted treatment, with implications for targeted treatment (Dugas & Robichaud, 2007; Dunsmoor et al., In press; van der Heiden, Muris, & van der Molen, 2012).
Here we used cued fear conditioning to assess the relationship between individual differences in self-reported IU and in psychophysiological correlates of fear extinction learning over time. We measured skin conductance response (SCR) and self-reported uneasiness whilst participants performed the conditioning task. We used an aversive sound as an unconditioned stimulus and visual shapes as conditioned stimuli, as in previous conditioning research (Barrett & Armony, 2009; Büchel, Morris, Dolan, & Friston, 1998; Delgado, Nearing, LeDoux, & Phelps, 2008; Neumann and Waters, 2006, Phelps et al., 2004). We hypothesized that, during fear extinction learning, future threat uncertainty sensitivity would predict generalized fear expression to both learned threat and safety cues, and/or sustained fear expression to learned threat cues (Morriss et al., 2015). Given that fear extinction paradigms are temporally sensitive (Gazendam et al., 2013, LaBar et al., 1998, Milad and Quirk, 2012, Phelps et al., 2004, Sehlmeyer et al., 2011), we expected this effect to be indexed by: (1) Larger responses in high IU individuals to both learned threat and safety cues in early fear extinction, across SCR and self-reports, and (2) sustained responses in high IU individuals to learned threat cues vs. safety cues during late fear extinction, across SCR and self-reports. Similar to our previous work (Morriss et al., 2015), we tested the specificity of the involvement of IU by comparing it with broader measures of anxiety, such as Spielberger State-Trait Anxiety Inventory, Trait Version (STAIX-2) (Spielberger et al., 1983) and Penn State Worry Questionnaire (PSWQ) (Meyer, Miller, Metzger, & Borkovec, 1990).
2. Method
2.1. Participants
38 students took part in this study (age range = 18–25 years; 32 females & 6 males). All participants had normal or corrected to normal vision and could only take part if they were in between 18 and 25 years of age. Participants provided written informed consent and received course credit for their participation. Participants were recruited through advertisements and the University of Reading Psychology Panel. The procedure was approved by the University of Reading Ethics Committee.
2.2. Procedure
Participants arrived at the laboratory and were informed on the procedures of the experiment. Firstly, participants were taken to the testing booth and given a consent form to sign as an agreement to take part in the study. Secondly, to assess emotional disposition we asked participants to complete a series of questionnaires presented on a computer in the testing booth. Next, physiological sensors were attached to the participants’ non-dominant hand. Participants were simply instructed to: (1) maintain attention to the task by looking and listening to the colored squares and sounds presented, (2) respond to the uneasiness scale that followed each trial (see “Conditioning task” below for details) using the keyboard with their dominant hand and (3) to sit as still as possible. Participants were presented a conditioning task on the computer, whilst electrodermal activity, interbeat interval and ratings were recorded. After the task, subjects were asked to rate the valence and arousal of the sound stimulus using 9-point Likert scales ranging from 1 (Valence: very negative; Arousal: calm) to 9 (Valence: very positive; Arousal: excited). All together, the experiment took approx. 1 h.
2.3. Conditioning task
The conditioning task was designed using E-Prime 2.0 software (Psychology Software Tools Ltd, Pittsburgh, PA). Visual stimuli were presented using a screen resolution of 800 × 600 with a 60Hertz refresh rate. Participants sat at approximately 60 cm from the screen. Sound stimuli were presented through headphones.
Visual stimuli were light blue and yellow squares with 183 × 183 pixel dimensions that resulted in a visual angle of 5.78° × 9.73°. The aversive sound stimulus consisted of a fear inducing female scream (sound number 277) from the International Affective Digitized Sound battery (IADS-2) and which has been normatively rated as unpleasant (M = 1.63, SD = 1.13) and arousing (M = 7.79, SD = 1.13) (Bradley & Lang, 2007). We used Audacity 2.0.3 software to shorten the female scream to 1000 ms in length and to amplify the sound by 15 db, resulting in a 90 db (∼5 db) sound. An audiometer was used before testing to standardize the sound volume across participants.
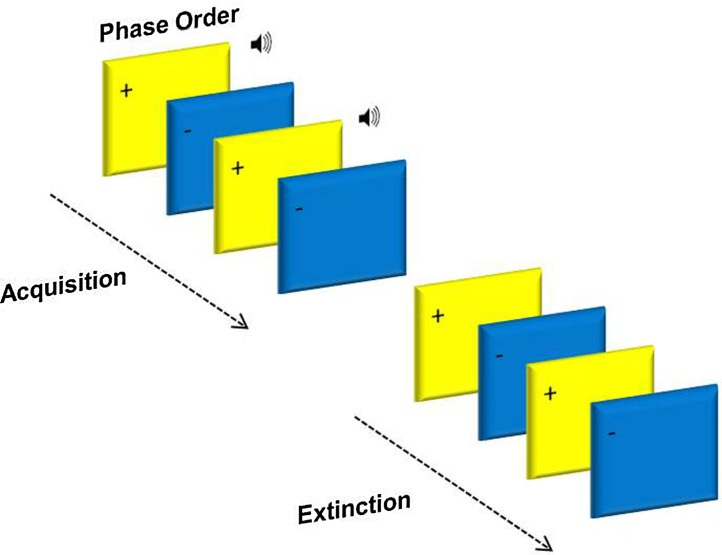
Acquisition and extinction phases were presented in two separate blocks (see Fig. 1). In acquisition, one of the squares (blue or yellow) was paired with the aversive 90 db scream 100% of the time (CS+), whilst the other square (yellow or blue) was presented alone (CS−). In extinction, both stimuli were unpaired (CS+, CS−). The third phase was a partial reacquisition, CS+ squares were paired with the sound 25% of the time, and the CS− remained unpaired (results not reported here).
The acquisition phase consisted of 24 trials (12CS+, 12CS−), the extinction phase 32 trials (16CS+, 16CS−) and the reacquisition 30 trials (16CS+ (4 unpaired), 14CS−; results not reported here). Experimental trials within the conditioning task were pseudo-randomized into an order, which resulted in no more than three presentations of the same stimulus in a row. Conditioning contingencies were counterbalanced, with half of the participants receiving the US with a blue square and the other half of participants receiving the US with a yellow square.
The presentation times of the task were: 1500 ms square, 1000 ms sound (played 500 ms after the onset of a CS+ square), 3000–6450 ms blank screen, 4000 ms rating scale, and 1000–2500 ms blank screen (see Fig. 1). The uneasiness rating scale asked how ‘uneasy' the participant felt after each stimulus presentation, where the scale was 1 ‘not at all' − 9 ‘extremely'.