Core Concept: How synaptic pruning shapes neural wiring during development and, possibly, in disease
By Jill Sakai
Letter 8 Resource: Link to Article
At birth, an infant’s brain is packed with roughly 100 billion neurons—some 15% more than it will have as an adult. As we learn and grow, our experiences strengthen the circuits that prove most relevant while the others weaken and fade.
“One extreme view of this would be that you start out wired up for every possible contingency,” says Jeff Lichtman, a neuroscientist at Harvard University in Cambridge, MA. Over time, a large percentage of those wires are permanently disconnected, says Lichtman. “What you're left with is a narrower nervous system,” he explains. “But it’s tuned exactly to the world you found yourself in.”
The process of elimination is key to forming a healthy, adaptive brain. Researchers have documented waves of neuronal cell death and the dramatic reduction of neurons’ connecting axon fibers early in neural development. But synapses, the fixed points where one cell’s axon exchanges signals with another cell, continue to be selectively removed at least through adolescence in humans, refining a coarse neural map into mature circuits.
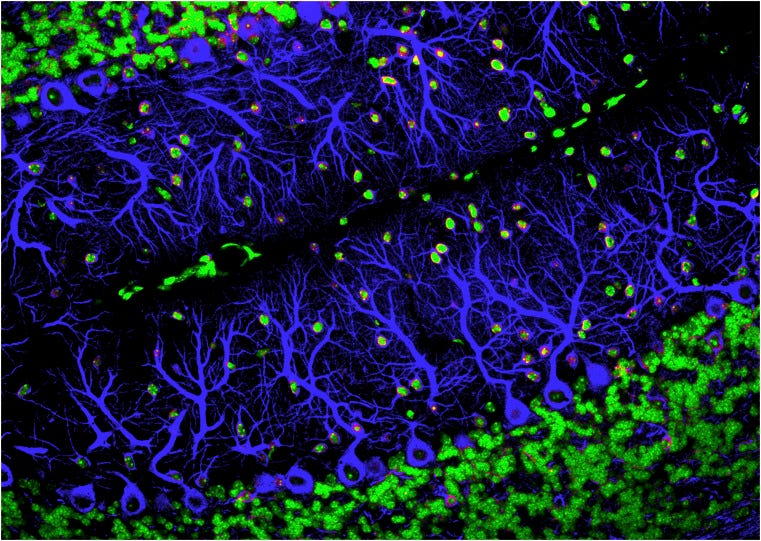
With improved imaging techniques and molecular tools, researchers are now exploring why synaptic pruning—the targeted elimination of functional synapses—happens and how it works. The amount and timing of neural activity are central to determining which synapses get reinforced and retained, and which get weaker—which flags them for destruction. Elements of the immune system appear to be essential to carrying out the elimination process. But researchers are also coming to recognize how pruning gone awry in children and teenagers could lay the foundation for neurological disorders, such as schizophrenia or autism. One possibility is that these are diseases of the wiring diagram—what Lichtman terms “connectopathies.” It may also be the case that some of the same pruning mechanisms that normally help refine brain wiring early in life contribute to later pathological synapse loss in dementia and other neurodegenerative disorders. If so, the pruning machinery could be a therapeutic target.
Selective Sculpting
Since the 1970s, neuroscientists have known that synaptic density in the brain changes with age. Peter Huttenlocher, a pediatric neurologist at the University of Chicago, IL, painstakingly counted synapses in electron micrographs of postmortem human brains, from a newborn to a 90-year-old. In 1979, he showed that synaptic density in the human cerebral cortex increases rapidly after birth, peaking at 1 to 2 years of age, at about 50% above adult levels (1). It drops sharply during adolescence then stabilizes in adulthood, with a slight possible decline late in life.
In 1983, psychiatrist Irwin Feinberg, then at the University of California at San Francisco, described the reduction in density as synaptic “pruning” (2). Much like pruning a rosebush, the removal of weaker structures reallocates resources to those remaining, allowing them to grow stronger and more stable, explains Carla Shatz, a neurobiologist at Stanford University in Palo Alto, CA.
At first, it might seem inefficient to create an excess of connections only to remove many of them later, says Alison Barth, a neuroscientist at Carnegie Mellon University in Pittsburgh, PA. In fact, computational biology suggests that selective pruning optimizes the brain’s circuits. In 2015, Barth and her colleagues used simulated neural networks to look at how synapse removal shapes network structure and function, comparing different rates and timing of elimination (3). Patterns like those measured in mouse and human brains—an initial period of rapid, aggressive elimination, followed by a slower decline—improved the capacity of the resulting network to carry information. “Networks that are constructed through overabundance and then pruning are much more robust and efficient than networks that are constructed through other means,” Barth says. “Evolution has selected for [these] properties of network construction,” a process she calls “incredibly beautiful.”
Similar patterns of excess connectivity followed by refinement show up throughout the nervous system, including the visual system, cerebellum, and neuromuscular junctions. In the 1980s, Shatz and her colleagues performed a series of seminal experiments in the visual system of cats. In the weeks before birth, a cat’s retinal cells send their axons to a brain structure called the lateral geniculate nucleus (LGN), where they branch diffusely and form synapses on many parts of the structure. Over time, most of those branches and synapses are pruned away as a select few grow elaborate arbors in a single region, laying out a map of visual space on the LGN (4, 5). But when the researchers blocked neural activity, the pruning failed. Without neural activity to trigger or guide pruning, the branches remained diffuse and overlapping, resulting in a jumbled visual map (6, 7).
“We showed that there was both a functional and structural basis for this activity-dependent synaptic remodeling,” Shatz says. Simultaneously, “the other synapses that were made in the right places became bigger and more beautiful and stronger.”
Surprising Role
With clear evidence that synaptic activity is what guides proper pruning, researchers’ attention turned to uncovering the cellular mechanisms that might regulate the remodeling. Cornelius Gross, a neurobiologist at the European Molecular Biology Laboratory in Rome, Italy, noted that mouse neurons upregulate a signaling molecule called fractalkine during synaptic maturation. Fractalkine signals to the brain’s resident immune cells, microglia, which are perhaps best known for their role in engulfing tagged pathogens and cellular debris during an immune response. “That gave us a clue,” Gross says. “The microglia might be doing something with the synapses.”
In a 2011 study, his team spotted synaptic material inside microglia, suggesting that the cells might play an active role in pruning synapses. Plus, disrupting fractalkine communication between microglia and neurons in an otherwise healthy mouse left brain circuits immature into adulthood, implicating microglia-mediated synaptic pruning as a critical step in refining the circuitry (8).
Just a few years earlier, neuroscientist Beth Stevens, then a postdoctoral fellow at Stanford University, had unexpectedly uncovered a similar role for a group of immune molecules, called complement proteins, in synaptic pruning on retinal axons (9). It came as a bit of a surprise. “This was a time where people weren’t really thinking about [innate] immune molecules doing these types of things in the healthy developing brain,” Stevens says.
In the immune system, complement proteins are thought to tag cell membranes, telling microglia what to engulf. So, Stevens suspected that there might be a link to the emerging role of microglia in synaptic remodeling. In 2012, her team at Boston Children’s Hospital, MA, found that, indeed, in the newborn mouse visual system, microglia can engulf synapses in the LGN in a process mediated by both complement and neuronal activity. And similar to Gross’ results with fractalkine, blocking those complement signals disrupted the developing visual circuits (10). Stevens and her colleagues proposed that complement molecules may tag low-activity synapses, marking them for microglial destruction.
It’s still not entirely clear what role the microglia play, however. With time-lapse imaging, Gross’ team recently watched microglia “nibbling” on presynaptic structures of live neurons in culture, a process called trogocytosis that immune cells use to sample, or even kill, pathogens and diseased cells (11, 12). But, he says, they didn’t find compelling evidence that the microglia actually engulf or prune full synapses. “They seem to nibble, but the nibble doesn't necessarily mean they’re eliminating. So, I think the jury's out. I don't think we know what they do there yet,” Gross says.
Read More: LINK TO FULL ARTICLE

